About the Viral Vector Characterization & Analytical Development Summit
As a wave of novel viral vector-based therapies enter pipelines enabling greater payload efficiency then seen before, the spotlight it on ensuring characterization and analytical development to enable economically viable capsid production at scale.
With it crucial analytical method development keeps to pace with pipeline progress, in this new era biopharma teams are faced with the urgent need to gain clarity across regulatory expectations and fit-for-purpose analytical technologies to define robust CQA’s. With the end goal to leverage analytics for faster process development read-out and optimization, there’s heightened awareness of the importance of characterizing viral vectors to demonstrate confidence of product quality for patient safety.
To address key bottlenecks and provide learnings across industry’s priorities, the Viral Vector Characterization & Analytical Development Summit took place with updates from AAV, LV & RV strategies for cell, gene & vaccine pipelines, to inform thinking for the best analytical development path forward with precision, accuracy, quality and efficacy optimized throughout.
Attendees joined peers at an exciting time for viral-vector based therapies, to execute analytical assays for enabling smooth transitions from bench to bedside.
Top 5 Actionable Takeaways You Missed in 2023:





Developing a robust and phase appropriate package development, with accuracy enabling vector efficacy optimization with Takeda & Adicet Bio
Defining predictive CQA’s with an informed understanding of evolving regulatory requirements to bring a systematic approach to studies with a clearer approval path forward with Ultragenyx
Leveraging advanced stability, safety & purity characterization insights to inform necessary process development and formulation optimization and speed programs with BioMarin Pharmaceuticals
Informing your choice of novel advanced analytical technologies and manage data interpretation effectively, for faster turnaround times, error avoidance and effective tech-transfer with Neogene Therapeutics & Moffitt Cancer Center
Optimizing quality consistency with greater physio chemical property understanding, empty vs. full measurements and impurity assessments to minimize risk with viral integrity preserved with Sanofi, Vertex Pharmaceuticals & Pfizer
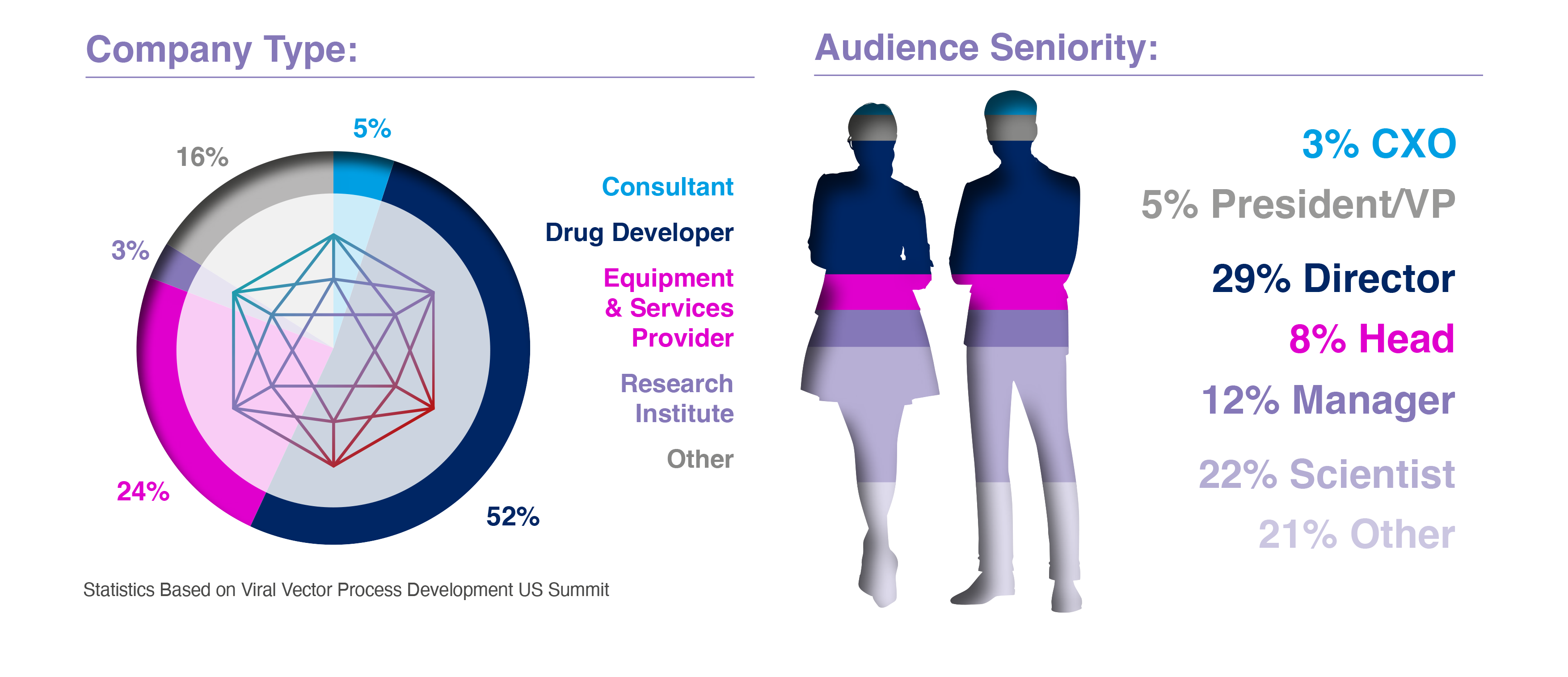
Audience Breakdown:
Head, Director, Scientists, leading:
- Viral Vector Analytical Development
- Viral Vector Characterization
- Vector & Capsid Engineering
- Viral Vector Quality
- Viral Vector Assay Development
- Viral Vector Process Development
- Viral Vector CMC & MSAT
- Viral Vector Upstream Process
From Biopharma & Leading Academics
